
-
Compliance with Confidence™
Compliance with Confidence™
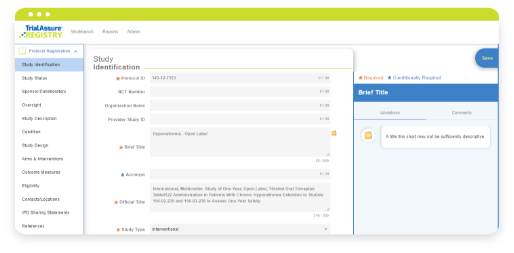
Learn MoreREGISTRY® is a powerful clinical trial disclosure and transparency reporting application designed to assist companies in navigating complex global clinical trial registration, clinical trial results disclosure, and understanding and ensuring compliance of those activities. Meet compliance and transparency goals through a scalable platform that regularly adapts to changing global requirements.
-
Analyze Global Transparency Compliance in One Place
Analyze Global Transparency Compliance in One Place
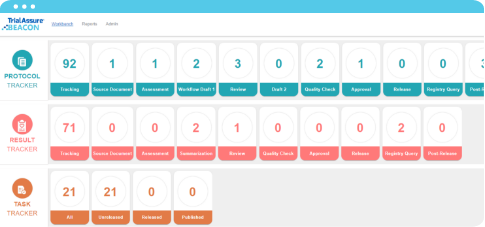
Learn MoreBEACON™ is your central, one-stop location to track global transparency compliance across the myriad of transparency requirements and guidelines, while ensuring public access to clinical trial information. BEACON’s flexible, built-in rules engine provides a simple intuitive dashboard interface showing status, including compliance, across all of your transparency activities. And, with custom configuration, BEACON incorporates your internal workflows, processes, timeframes, and internal benchmarks.
-
Medical Writing AI Technology for the Pharmaceutical Industry

Medical Writing AI Technology for the Pharmaceutical Industry
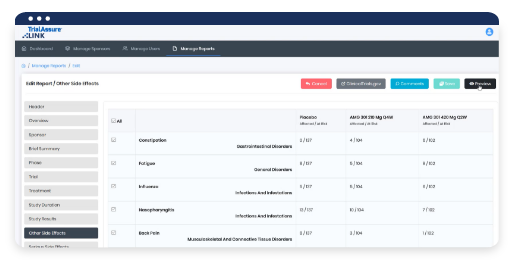
Learn MoreLINK® AI helps you efficiently develop, translate, and create drafts of clinical, technical, and plain language documents to meet increasing resource demands and transparency compliance requirements across the drug development lifecycle. At the click of a button, users can create a draft of routine clinical, regulatory, plain language, and other scientific documents.
-
Anonymize Trial Data and Documents Simply and Efficiently
Anonymize Trial Data and Documents Simply and Efficiently
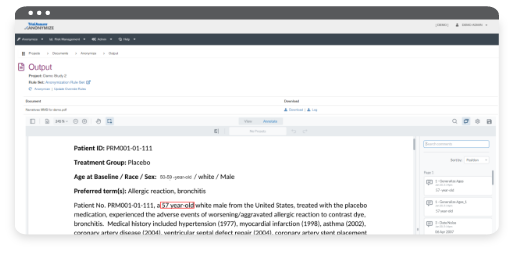
Learn MoreANONYMIZE® for data and document anonymization and redaction empowers you to responsibly share clinical trial data and information with confidence. With ANONYMIZE, you have the unique ability to maintain the integrity of clinical trial data and information, facilitate secondary research, and employ state-of-the-art security to ensure the privacy of clinical trial participants and Sponsors alike. ANONYMIZE is entirely configurable to your specifications and it is architected for scale and the future of transparency, including the ability to leverage and utilize Machine Learning, Natural Language Processing, and Artificial Intelligence.





