For the first time in US history, the FDA has taken action against a clinical trial Sponsor for non-compliance with ClinicalTrials.gov requirements.
After a Pre-Notice of Noncompliance, the FDA decided to issue its first notice of non-compliance against a Sponsor. If the Sponsor doesn’t comply within 30 days, the FDA can apply $10,000 in fees for each day of non-compliance.
Alltrials and the TrialsTracker puts pressure on sponsors
For several years, clinical transparency experts alerted Sponsors about these possible actions. In the last five years, there were several signs that the FDA would soon apply this policy.
Back in 2016, Alltrials, led by Dr. Ben Goldacre, launched the FDAAA Trials Tracker.
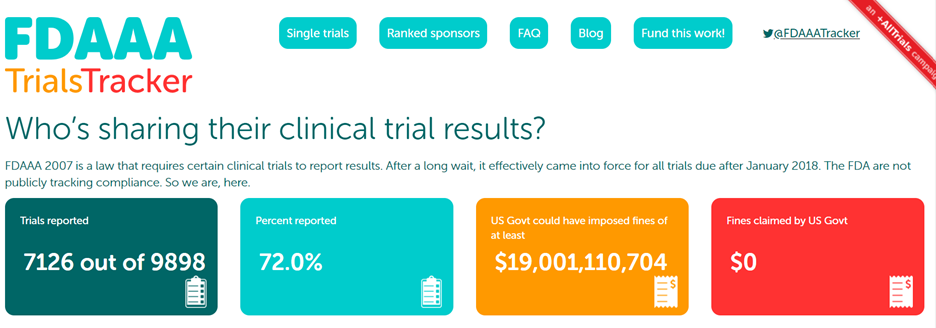
This US tracker identifies clinical trials from major Sponsors and assesses the percentage of unpublished clinical trial results in clinicaltrials.gov. The first warning is given, and it is tough to ignore for those working in clinical trials.
Following the success of this tracker, Alltrials developed a similar version, known as the EU Trials Tracker, for the European register.
FDA initiates a reflection on law enforcement
In the United States, FDAAA section 801 provides for financial penalties of up to $10,000 per day of delay, 30 days after a notice of non-compliance. But again, this sanction was not applied for many years.
The first effect that the FDAAA Trials Tracker had on transparency was the publication by the FDA in 2017 of draft guidance outlining how non-compliance will be identified and punished. However, this guidance remained in the draft version for many years.
In Europe, according to the guidance of the European Commission, the theoretical sanction is to be publicly flagged after three months of delays. However, the sanction has never been applied.
The effect of the Alltrials launching the FDAAA Trials Tracker
AllTrials began using a financial argument of how much money the FDA could have earned through fines to convince public authorities to apply the law. In February 2018, Alltrials wrote an open letter to the FDA commissioner calling for the law’s enforcement.
At the same time, Alltrials continued the use of the FDAAA TrialsTracker, which focuses on organizations that do not post their results under the terms of FDAAA section 801. To really hammer home the point, the FDAAA tracker even presents the number of fines that the US Government could have imposed if they enforced the law.
The FDAAA TrialsTracker has a strong psychological effect on sponsors, but it will not be the key element in shifting FDA policy.
A federal judge requires FDA to enforce the FDAAA Section 801
In February 2020, a tipping point occurred. The decision of a federal judge, reported by STAT News, has a strong impact on the FDA law enforcement policy.
Peter Lurie, former associate FDA commissioner well versed in transparency issues, and Charles Seife, a New York University journalism professor, sued the FDA to enforce the law. A Federal judge partially ruled in their favor and required that sponsors publish the results of all interventional trials started since 2007 for studies that:
- Have a clinical trial site in the US
- Are included in the Investigational New Drug Application (IND)
- Have at least one manufacturing step in the US
In the above mentioned STAT article, the FDA spokesman’s reaction was very restrained: “the agency is evaluating the Court’s decision with the Department of Justice to determine our next steps.”
FDA got the message
Six months after the trial, the draft guideline shifted to a final version.
In December 2020, ClinicalTrials.gov then included functionality in the advanced search to find all companies flagged for an FDAAA Section 801 violation.
Now, in May 2021, we are witnessing the first FDA notice of non-compliance. And, judging by the information from the FDAAA Trials Tracker, it might apply to many more.
The control of compliance with FDAAA Section 801 can intervene at two stages:
- During the inspection of a clinical trial: The inspectors will use a checklist to identify whether the study is in compliance
- After complaints from the public: Given the activity of public watchdogs, one should expect to see more and more of this type of situation
Therefore, it is strongly recommended to include clinical trial disclosure within the clinical trial process and to correct the situation if the organization finds deviations from FDAAA Section 801.
The situation in Europe
The EMA recently confirmed the entry into force of the European Clinical Trials Regulation in January 2022. This Regulation allows every Member State to decide financial and penal sanctions in case of unpublished studies.
In France, for instance, in accordance with the article L1126-12 of the Public Health Code, any delay in the publication of the results of a clinical study can trigger a jail sentence for up to one year and a fine of 15,000 Euro. This article is also applicable for any delay in publishing lay summaries.
Transparency, therefore, becomes an essential activity that must be taken seriously by Sponsors at the risk of receiving serious consequences.
This article was written by Karim Ibazatene, Associate Director, Clinical Trial Transparency.

