Although penalties for Responsible Parties who failed to comply with clinical trial registration or results submission requirements were written into FDAAA 801, these penalties, in the form of civil monetary penalties and withholding of grant funds for federally funded studies, were never levied.
The Final Rule outlines similar potential legal consequences for non-compliance, but it adds a provision to identify “non-compliant” postings as such on ClinicalTrials.gov. The Food and Drug Administration (FDA) has announced the availability of a final guidance entitled “Civil Monetary Penalties Relating to the ClinicalTrials.gov Data Bank: Guidance for Responsible Parties, Submitters of Certain Applications and Submissions to FDA, and FDA Staff” in which they outline their current thinking on levying civil monetary penalties related to not registering protocols and submitting results to ClinicalTrials.gov.
Guidance for Civil Money Penalties
Section 303(f)(3) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) authorizes the FDA to assess civil money penalties against responsible parties and/or submitters of certain applications and submissions to FDA regarding drug products, biological products, and device products who violate applicable FD&C Act prohibitions relating to requirements under section 402(j) of the Public Health Service Act (42 U.S.C. 282[j]), including its implementing regulations in 42 CFR Part 11, to submit registration and/or results information to the ClinicalTrials.gov data bank and/or certain certifications to FDA.
This guidance addresses the following key questions:
- How will the parties who fail to submit required protocol registration and results information to ClinicalTrials.gov be identified?
- When (under what circumstances) will the FDA levy civil monetary penalties against a responsible party?
- How will the FDA seek civil monetary penalties?
- What civil monetary penalty amounts can be assessed for:
Identification of Violations
Violations relating to ClinicalTrials.gov will be identified through (a) evidence collected during Bioresearch Monitoring Program (BIMO) inspections conducted per the FDA, or (b) based on evaluation of complaints received by the Agency. BIMO inspections are typically conducted in association with the submission of a research or marketing application or are a part of the Agency’s investigation of a complaint. Potential violations include: (a) failing to submit required clinical trial registration and/or results information to ClinicalTrials.gov; (b) submitting false or misleading information to ClinicalTrials.gov; (c) failing to submit the required certification to the FDA; and (d) knowingly submitting false certification to the FDA.
Process
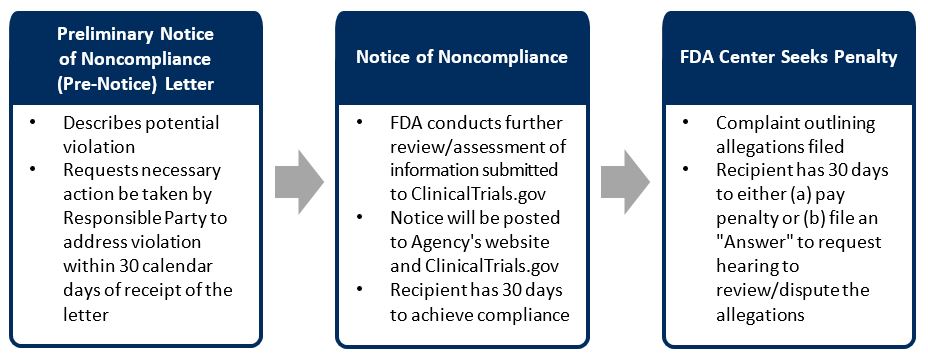
How Will the FDA Seek Penalties?
If civil monetary penalties are sought, the FDA Center will file a Complaint and serve the Responsible Party. The Complaint will outline the allegations, the violations leading to the allegation, as well as the amount of the penalties being sought.
The Complaint will also outline the process whereby the Responsible Party can file an Answer to request a hearing to review/dispute the penalties, and a warning that the proposed penalties will be sought if an Answer is not received within 30 days of the receipt of the Complaint.
Civil Monetary Penalty Amounts
The maximum monetary penalties under Section 303(f)(3)(A) of the FD&C Act are not to be more than $10,000 for all violations adjudicated in a single proceeding. If violations aren’t corrected within 30 days of notification, Section 303(f)(3)(B) of the FD&C allows for an additional penalty of $10,000 for each day that the violation is not corrected.
When determining the amount of the penalties, the following factors are considered: “the nature, circumstances, extent, and gravity of the violation(s) and, with respect to the violator, ability to pay, effect on ability to continue to do business, any history of prior such violations, the degree of culpability and such other matters as justice may require.”
Ask Kasim any questions you have by filling out this form.

